Trying to get informed consent
The recent “Why do you overeat?” note shared something that happened 20 years ago. This week’s note shares something that happened three years ago. I hesitated before sharing this, as I know that the topic is emotive; but the exchange is interesting and important and so I decided to share it with you.
Introduction
I first started researching vaccines for coronaviruses in spring/summer 2020 when it became obvious that lockdowns were not going to be “three weeks to flatten the curve.” It became clear that it was intended that we should remain in lockdown until a vaccine was developed. I cannot convey how much that alarmed me. Everything that was happening at that time was in conflict with my core values and my response was visceral.
My first post on Covid-19, on March 20th, 2020, reported that coronaviruses were first identified in the mid-1960s (Ref 1). We had thus had coronaviruses for over 50 years. My research quickly revealed that we had developed no vaccines during that time. This appeared to be because of something called “Antibody dependent enhancement”, which showed up in animal testing. In brief, this meant that the animals developed antibodies following vaccination (which was good), but they then had a worse response than unvaccinated controls when exposed to the virus (which was bad).
Lockdown was announced in the UK on Monday March 23rd, 2020. The UK Astra Zeneca Covid-19 vaccine trial was registered three days earlier on 20th March 2020 (Ref 2). I didn’t know that at the time; it would have shocked me. The trial registration submitted full plans for 19 different arms/interventions. These included the product to be injected (ChAdOx1 nCoV-19), the dose(s), the placebo (a meningitis vaccine), the timetable, the locations, the number of participants, inclusion and exclusion criteria etc. The speed at which products were moving from laboratories to arms was impressive to many and concerning to some (one could be both impressed and concerned). I was aware that the Astra Zeneca (AZ) trial was already underway as early as spring 2020, as I knew one participant. When the first trial papers were published (December 2020), it was confirmed that recruitment for the AZ trial had started from April 23rd, 2020 (Ref 3).
In October 2020, Dr Peter Doshi’s important paper was published in the BMJ (Ref 4). This paper analysed seven vaccines in development and what they were designed to test. Doshi reported that none of the vaccine trials were designed to test for either transmission or severity of outcome. The two things that we most wanted to know – will vaccines stop spread and will they provide protection against bad outcomes – were not even being tested. The only outcome of interest was – did the trial participant test positive on a PCR test, which was a highly unreliable measure.
In December 2020, the outcome papers for the AZ and Pfizer trials were published in The Lancet and the NEMJ respectively. I examined both here (Ref 5). Both vaccines were approved for use in the UK that month. Pfizer approval followed in more countries quickly. The AZ product was less readily adopted. On December 8th, 2020, the first member of the public (as opposed to a trial volunteer) was given the Pfizer vaccine (Ref 6).
The trial protocols were disregarded from the outset. The December 2020 outcome paper for the AZ trial was a summary of four sub trials – two in the UK (COV001 and COV002); one in Brazil (COV003) and one in South Africa COV005. In COV001 alone, there were four intervention groups and protocols (all vs the meningitis vaccine placebo): Group 1 single dose; Group 2 booster at 8 weeks; Group 3 two doses 10 weeks apart; Group 4 single dose but with paracetamol (Ref 7). The Pfizer protocol that was trialled was one jab followed by a second three weeks (21 days) later. The UK roll out started with a 12 week gap between the 2 injections. This was rationalised as “let’s give more people some protection”, but the 12 week protocol had never been tested before mass global roll out (Ref 8).
I didn't give much thought to the injections over the winter of 2020-2021 as they weren't intended for me. The message was 15 million jabs to freedom (Ref 9). The promise was that once the over 65s and younger people with comorbidities had been jabbed, we would all be released from house arrest. When that didn't happen, my visceral fear reached a different level. The new message became "no one is safe until everyone is safe." But I knew that the injections didn't stop someone getting Covid, they didn't stop transmission of Covid, and they didn't reduce severity of outcome (the latter two not even having been tested). As 2021 progressed, the narrative became more and more sinister. Soon pro-vaccine people who had had every vaccine throughout their lives were being called anti-vaxxer for having some doubts about this one (Ref and note 10). Soon after that, people were being refused entry to countries and venues and fired from jobs if they didn’t want this novel product.
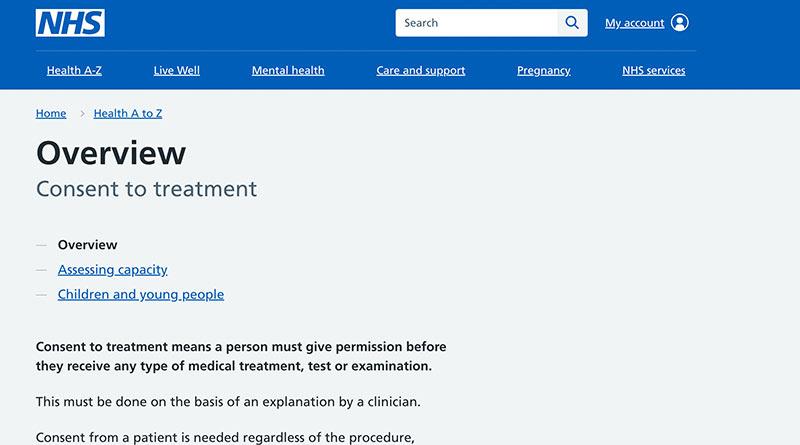
Voluntary and informed consent
The UK National Health Service (NHS) principle of informed consent states (Ref 11): “For consent to be valid, it must be voluntary and informed, and the person consenting must have the capacity to make the decision. The meaning of these terms are:
voluntary – the decision to either consent or not to consent to treatment must be made by the person, and must not be influenced by pressure from medical staff, friends or family;
informed – the person must be given all of the information about what the treatment involves, including the benefits and risks, whether there are reasonable alternative treatments, and what will happen if treatment does not go ahead;
capacity – the person must be capable of giving consent, which means they understand the information given to them and can use it to make an informed decision.”
Informed consent was abandoned during this period of medical history. Read those definitions of both “voluntary” and “informed” carefully. Regarding “voluntary”, pressure was exerted from the Queen of England to the prime minister of New Zealand to the president of the US, with every world leader in between. Medical staff, friends and family all exerted pressure, as did celebrities, neighbours, employers and social media ‘influencers.’ The denial of rights to travel, to enter venues and even to remain in employment were in stunning contravention of the voluntary aspect of informed consent.
Regarding “informed”, this is what happened when I tried to obtain information about the benefits and risks of the treatment…
My invitation
By spring 2021, Wales was rattling through the administration of injections. On March 11th, 2021, I received an invite from Aneurin Bevan University Health Board (ABUHB) to attend Cwmbran sports stadium to receive a Covid vaccine. I was given a date and time (24th March 14.45pm). This was clever. A number of people I spoke to attended because they didn’t want to miss an appointment and ‘let the NHS down’ when the NHS was so busy.
I chose instead to write to the Chief Executive (Judith Paget CBE ) and Chair (Ann Lloyd CBE) of Aneurin Bevan University Health Board as follows:
My letter
Dear Ms Lloyd, Ms Paget (sent separately to each),
Thank you for your kind invitation for me to receive a Covid-19 vaccine.
Both the Pfizer BNT162b2 mRNA Covid-19 vaccine and the ChAdOx1 nCoV-19 Oxford/ AstraZeneca vaccine are novel drugs. Both drugs have only been approved for emergency use. The trials are ongoing, with the Pfizer trial not due for completion until January 31st, 2023 (Ref 12) and the Oxford/AstraZeneca trial not due for completion until February 14th, 2023 (Ref and note 13).
The NHS policy is “for consent to be valid, it must be voluntary and informed” (Ref 14). Given the novelty, rapid development and incomplete trial history of these interventions, consent is vital. For my consent to be first informed, and second voluntary, please can you answer the following:
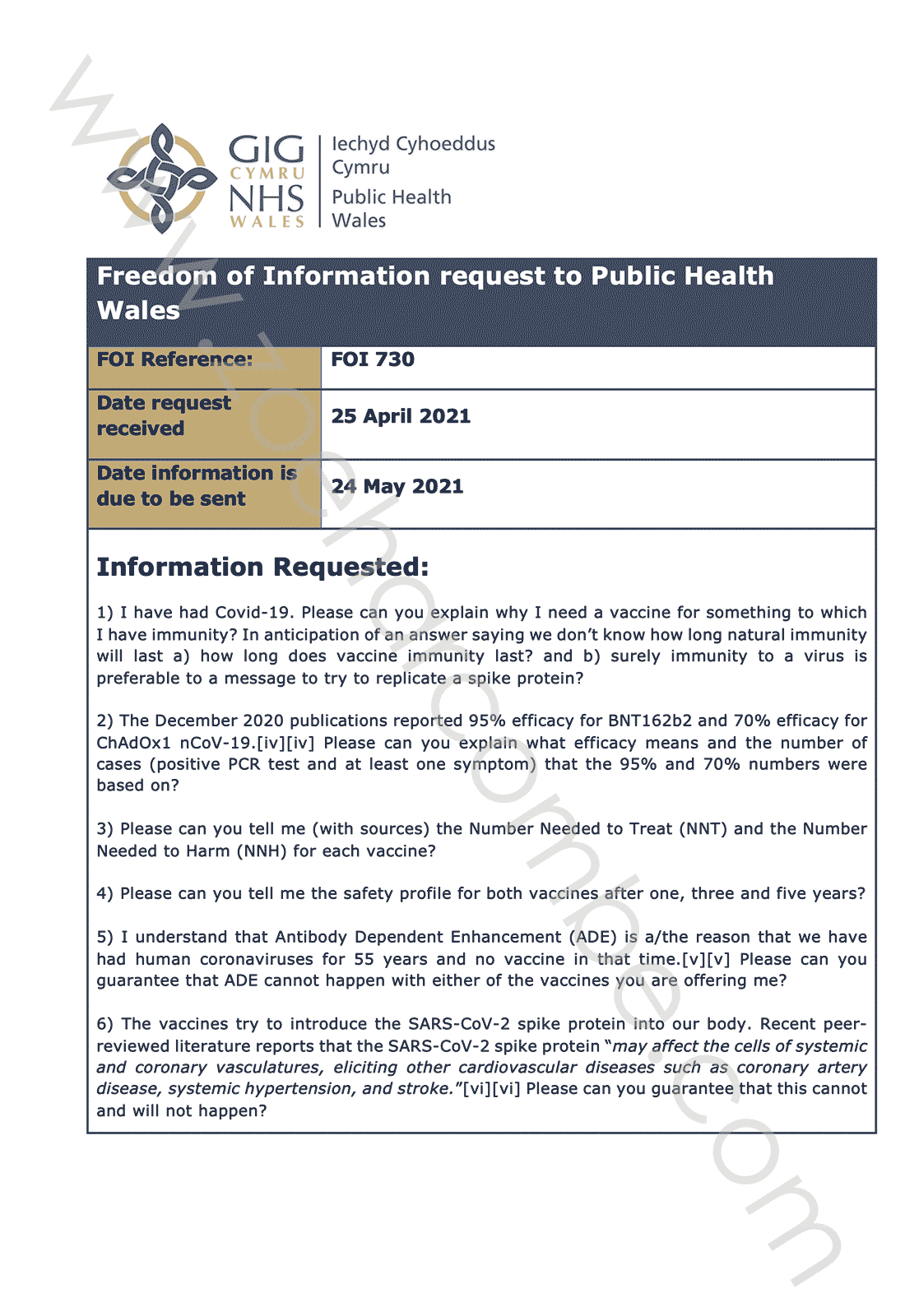
1) I have had Covid-19. Please can you explain why I need a vaccine for something to which I have immunity? In anticipation of an answer saying we don’t know how long natural immunity will last a) how long does vaccine immunity last? and b) surely immunity to a virus is preferable to a message to try to replicate a spike protein?
2) The December 2020 publications reported 95% efficacy for BNT162b2 and 70% efficacy for ChAdOx1 nCoV-19 (Ref 15). Please can you explain what efficacy means and the number of cases (positive PCR test and at least one symptom) that the 95% and 70% numbers were based on?
3) Please can you tell me (with sources) the Number Needed to Treat (NNT) and the Number Needed to Harm (NNH) for each vaccine?
4) Please can you tell me the safety profile for both vaccines after one, three and five years?
5) I understand that Antibody Dependent Enhancement (ADE) is a/the reason that we have had human coronaviruses for 55 years and no vaccine in that time (Ref 16). Please can you guarantee that ADE cannot happen with either of the vaccines you are offering me?
6) The vaccines try to introduce the SARS-CoV-2 spike protein into our body. Recent peer-reviewed literature reports that the SARS-CoV-2 spike protein “may affect the cells of systemic and coronary vasculatures, eliciting other cardiovascular diseases such as coronary artery disease, systemic hypertension, and stroke” (Ref 17). Please can you guarantee that this cannot and will not happen?
7) Given that the vaccine manufacturers have indemnity from providing compensation if something goes wrong, please can you confirm that I can sue the board and individual members directly, with unlimited liability, if I am harmed in any way?
I am one of the rare 1% of people who follow all five healthy behaviours associated with reduced mortality (Ref 18). I don’t smoke. I don’t drink alcohol. I exercise daily. I have maintained a BMI of 20 for many years. I eat an optimally nutritious diet. My health is of utmost importance to me. I will not risk the huge effort I dedicate to my health without fully understanding what risk I am taking. Especially when I can discern no benefit whatsoever in me taking that unknown risk.
Thankyou
Yours sincerely,
Dr Zoë Harcombe
The questions were a mix of ones to which I knew the answer, but I wanted to know if the health board did and ones to which there was no answer and I wanted to know if the health board would admit this. Please note that I was questioning the cardiovascular impact of the Covid products as early as March 2021. Cardiac issues are now acknowledged but dismissed as rare or mild (there is no such thing as mild myocarditis). Many people claim that we know more now than we did then. We do; but we knew enough then. The concerns about mRNA technology were there from the outset.
My follow-up
On 6th April 2021, I needed to chase for a reply. I resent the two letters with a handwritten note at the top saying “Dear Ms Lloyd, Ms Paget (sent separately), I am being chased for my vaccine, so I need to chase you for my reply please. Thank you. Zoë.” I also submitted a Freedom of Information request (FOI) to the health board in parallel.
The first Health Board reply
On 7th April 2021, I received an email from ABUHB corporate services acknowledging receipt of the FOI request. I have no concerns about sharing this exchange, since FOI requests, by definition, are supposed to be freely available:
“Dear Dr Harcombe
“Thank you for your request for information under the Freedom of Information Act received on 6th April 2021. We have allocated it the following reference number FOI 21-161 and will be in contact again shortly. We aim to respond to all Freedom of Information requests within 20 working days from the date of receipt.”
The full Health Board reply
On 14th April 2021 I received an email with the response to the FOI request:
“Dear Dr Harcombe
“Thank you for your request for information under the Freedom of Information Act, received on 6th April 2021.
“Please find attached the Health Board’s response to this request.”
The attachment was as follows:
 <br/ >
<br/ > 
As you can see, the reply did not attempt to answer any of my questions. It said that ABUHB was delivering the Welsh government strategy and following guidance from other public bodies. In essence, the ABUHB position was "Nothing to do with us." The reply invited me to ask Public Health Wales if I had further questions. Further questions? I didn't have answers to my opening questions.
Involving Public Health Wales
On 19th April 2021 I emailed Public Health Wales. The title of the email was “A request for a review of JP/lab/FOI 21-161.”
The email said:
Dear Richard Howells, Board Secretary
I would like to request a review of a recent FOI that I submitted.
I was pleased with the speed of response, but not with the answers.
On March 16th, when I received an invitation for a Covid-19 vaccination, I sent the same letter to Judith Paget CBE, Chief Executive, and Ann Lloyd CBE, Chair. The letter asked seven questions, which I needed answering before I could accept the invitation. A copy is attached (the word file).
When I was chased for a jab, on April 6th I chased for replies. I submitted an FOI on April 6th in parallel. The FOI was the one responded to – although it wasn't. The FOI reply is also attached (PDF). None of my seven questions was answered. The only response given was that, in essence, "Aneurin Bevan University Health Board is following Welsh government and Public Health Wales orders." That may be the case, but these questions need answering please. The clinical trials for these injections do not complete until 2023. The Pfizer trial completion date has slipped since my first (March 16th) letter. It is now April 6th, 2023.
These are entirely reasonable questions to ask before taking part in a clinical trial with a novel drug with novel technology and known issues. Arguably you should not be vaccinating tens of thousands of people in ABUHB alone (two-three million across Wales) without knowing the answers to these questions.
If anything does go wrong (see Swine Flu), "following orders" would not be a defence.
I look forward to hearing from you.
Thank you.
Yours sincerely - Zoë
The attached letter said:
Dear Public Health Wales,
Following an invitation for a Covid-19 vaccination, I asked the following questions of my health board – Aneurin Bevan University Health Board. They have not answered them, but instead replied that "The Health Board follows all national guidance provided by the Welsh Government, JCVI and other relevant regulatory bodies and specialist advice regarding the vaccines is provide by Public Health Wales... Should you have any further questions regarding the JCVI advice or vaccinations please contact Public Health Wales directly."
And so I am. Please can you answer the following questions.
(The rest of the letter reiterated the seven questions).
Thank you.
Yours faithfully
Dr Zoë Harcombe, PhD
The reply from Public Health Wales
I had to chase this too. The first response from Public Health Wales was an auto reply saying (paraphrased) “Is your request really necessary? We’re dealing with a pandemic.”
On April 25th, 2021, I replied (verbatim) “I would like my FOI to be answered please. It is core to current issues and so cannot wait until the current issues are over.”
On May 11th, 2021, I received an email reply from Public Health Wales with an attached letter. The letter is copied below.
 <br/ >
<br/ >  <br/ >
<br/ > 
Again, none of my questions were answered. Again authority was deferred to. I had reached the end of the road to try to obtain the informed bit of informed consent, so I left it there.
I had asked reasonable and important questions and they had not been answered. I could not therefore give voluntary and informed consent to a novel intervention. What happened thereafter will forever horrify me.
Postscript
I happened to come across Judith Paget again in October 2023. Former Office of National Statistics (ONS) statistician, James Freeman, tweeted a letter that had been sent to him (Ref 19). The letter was from Padget, and it was to all chief executives of all NHS Wales organisations. You can see the letter in the tweet. It was demanding to know what interventions would be made to overcome NHS staff "reluctance" to have more Covid-19 vaccines. This would be at least the fifth jab, if staff had accepted all invitations until then. I suspect, at the time of this demand, Padget still did not know the answers to my questions.
References
Ref 1: https://www.zoeharcombe.com/2020/03/coronavirus-covid-19-some-facts-figures/
Ref 2: https://clinicaltrials.gov/study/NCT04324606
Ref 3: https://www.zoeharcombe.com/2020/12/chadox1-ncov-19-the-lancet-papers/
Voyset et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. The Lancet. Dec 2020.
Ref 4: Doshi. Will covid-19 vaccines save lives? Current trials aren’t designed to tell us. BMJ. October 2020. https://www.bmj.com/content/371/bmj.m4037
Ref 5: https://www.zoeharcombe.com/2020/12/chadox1-ncov-19-the-lancet-papers/
Ref 6: https://www.england.nhs.uk/2020/12/landmark-moment-as-first-nhs-patient-receives-covid-19-vaccination/
Ref 7: Folegatti et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind, randomised controlled trial. The Lancet 2020. (See the supplementary file for group protocols).
Ref 8: https://www.imperial.ac.uk/news/243032/delaying-second-doses-vaccine-reduced-covid-19/
Ref 9: https://www.mailplus.co.uk/news/45502/countdown-to-15-million-jabs...-and-freedom-day
Ref and note 10: The definition of vaccine was changed to accommodate the new viral vector and mRNA products. The new technology is more accurately called gene therapy. Stefan Oelrich, board member and head of the pharmaceuticals division of Bayer, said at the opening ceremony of the World Health Summit 2021, "Ultimately the mRNA vaccines are an example for that cell and gene therapy... If we had surveyed, 2 years ago, in the public, would you be willing to take gene or cell therapy and inject it into your body, we would have probably had a 95% refusal rate."
https://www.youtube.com/watch?v=OJFKBritLlc
(the short clip is here https://twitter.com/zoeharcombe/status/1608734640436826118)
Ref 11: https://www.nhs.uk/conditions/consent-to-treatment/
(The following 7 references were the original 7 references in the letter to ABUHB. The notes that I have added to the original references for this post are in red.)
Ref 12: https://clinicaltrials.gov/ct2/show/NCT04368728 (This link currently shows trial completion was 10/2/2023).
Ref and note 13: https://clinicaltrials.gov/ct2/show/NCT04324606 (This link currently shows trial completion estimated to be 31/3/2024). Strictly speaking the AZ trial ended in the summer of 2020. Participants had been allocated to either the drug or placebo ‘blinded’ i.e., not knowing which one they had. COV001, COV002, and COV003 were single blinded (the researchers knew who was in each group but the participant didn’t). COV005 was double blinded – neither knew. Late summer 2020, I recall the participant I knew being told whether she was in the drug or placebo group so that she could take the drug if she had been given the placebo. This means that the trial was effectively over in its ability to compare drug with placebo within a few months of the trial starting.
Unblinding was reported to have happened in December 2020. https://www.telegraph.co.uk/global-health/science-and-disease/oxford-set-tell-vaccine-trial-volunteers-got-placebo-offered/
Ref 14: https://www.nhs.uk/conditions/consent-to-treatment/#
Ref 15: Polack et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. NEMJ. Dec 2020.
Voyset et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. The Lancet. Dec 2020.
Ref 16: Wen Shi Lee et al. Antibody-dependent enhancement and SARS-CoV-2 vaccines and therapies. Nature Microbiology. Sept 2020. https://www.nature.com/articles/s41564-020-00789-5.
Ref 17: Suzuki & Gychka. SARS-CoV-2 Spike Protein Elicits Cell Signaling in Human Host Cells: Implications for Possible Consequences of COVID-19 Vaccines. Vaccines. January 2021.
Ref 18: Li et al. Healthy lifestyle and life expectancy free of cancer, cardiovascular disease, and type 2 diabetes: prospective cohort study. BMJ 2020.
Elwood et al. Healthy lifestyles reduce the incidence of chronic diseases and dementia: evidence from the Caerphilly cohort study. PLoS One. 2013.
Ref 19: https://twitter.com/james_freeman__/status/1718656886944203065
The rest of this article is available to site subscribers, who get access to all articles plus a weekly newsletter.
To continue reading, please login below or sign up for a subscription. Thank you.